AI-Powered Productivity
Turn complaint handling into action, not admin.
Capture, classify, and review complaints in one system while preserving oversight and regulatory accountability across global vigilance requirements.
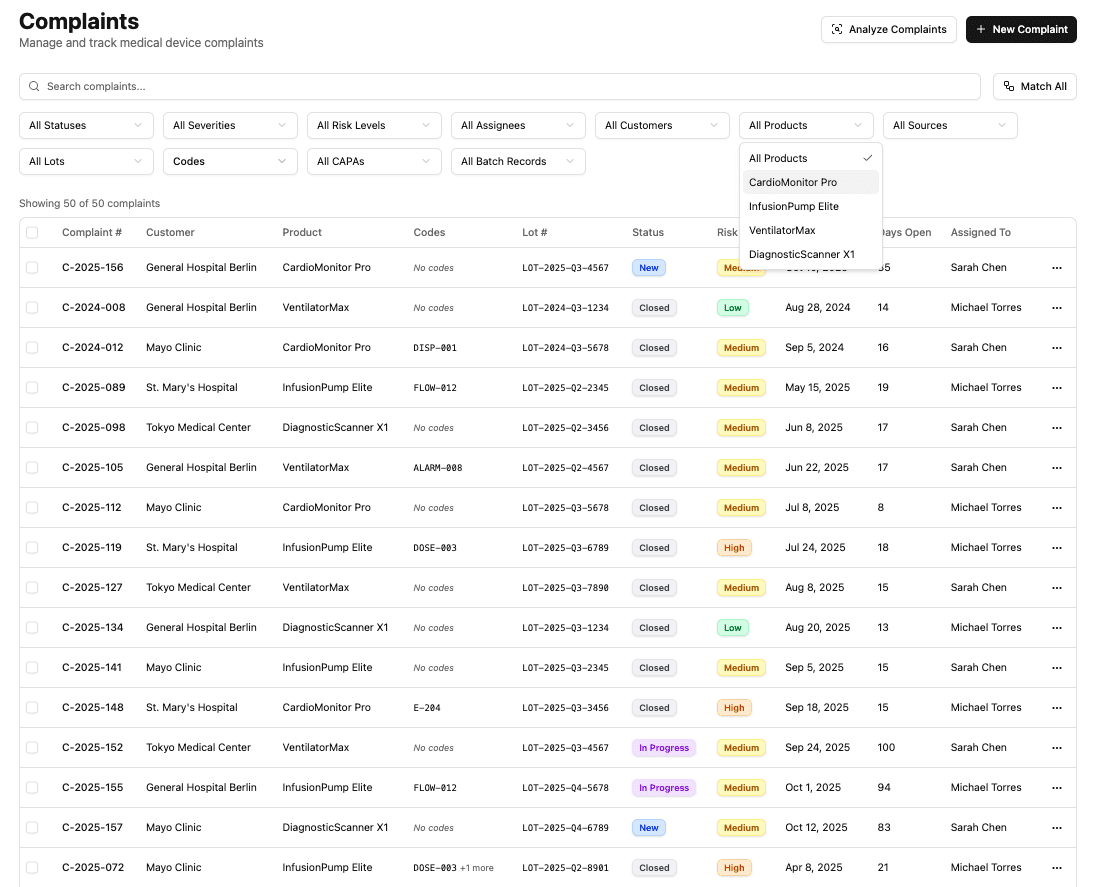
Centralize complaint intake without changing existing systems.
One connected system
Capture complaints from everywhere
Cytodyme sits on top of the systems your team already uses, pulling complaint data into a single operational view without disrupting intake workflows.
Multi-Source Intake
Pull complaints from tools your team already uses today.
Multi-Source Intake
Pull complaints from tools your team already uses today.
Multi-Source Intake
Pull complaints from tools your team already uses today.
Unified complaint view
See all complaints in one structured, searchable workspace
Unified complaint view
See all complaints in one structured, searchable workspace
Unified complaint view
See all complaints in one structured, searchable workspace
Live filtering and search
Filter by product, lot, status, source, or assigned owner
Live filtering and search
Filter by product, lot, status, source, or assigned owner
Live filtering and search
Filter by product, lot, status, source, or assigned owner
System of record preserved
Original complaint sources remain unchanged and auditable
System of record preserved
Original complaint sources remain unchanged and auditable
System of record preserved
Original complaint sources remain unchanged and auditable





AI-assisted coding
AI-assisted coding
AI-assisted coding
Reduce variability in coding and classification.
Instead of manually searching code lists and relying on individual judgment, Cytodyme assists teams by suggesting consistent codes with documented reasoning.
AI-assisted coding
Suggest complaint codes based on description content
AI-assisted coding
Suggest complaint codes based on description content
AI-assisted coding
Suggest complaint codes based on description content
Multiple code systems
Support IMDRF and MedDRA classification schemes
Multiple code systems
Support IMDRF and MedDRA classification schemes
Multiple code systems
Support IMDRF and MedDRA classification schemes
Confidence-scored suggestions
Display confidence levels to guide human review
Confidence-scored suggestions
Display confidence levels to guide human review
Confidence-scored suggestions
Display confidence levels to guide human review
Human approval required
Final coding decisions always require user confirmation
Human approval required
Final coding decisions always require user confirmation
Human approval required
Final coding decisions always require user confirmation
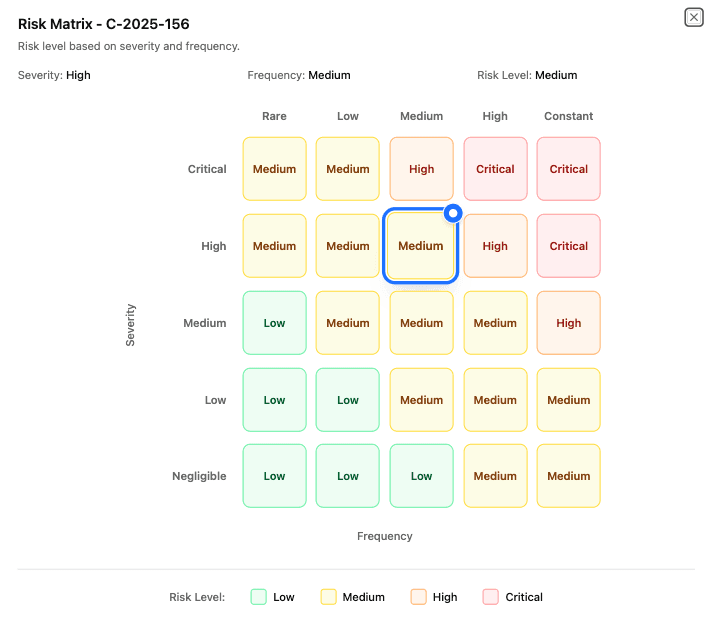
Real-time risk monitoring
Real-time risk monitoring
Real-time risk monitoring
See risk as it develops
Surface emerging safety issues as complaint patterns shift over time, not months later during review cycles. Cytodyme continuously updates severity and frequency so teams and managers can act with current information.
Live risk signals
Severity and frequency update as new complaints arrive
Live risk signals
Severity and frequency update as new complaints arrive
Live risk signals
Severity and frequency update as new complaints arrive
Configurable risk matrix
Apply organization-specific risk rules
Configurable risk matrix
Apply organization-specific risk rules
Configurable risk matrix
Apply organization-specific risk rules
Pattern escalation
Flag rising trends before they cross reportable thresholds
Pattern escalation
Flag rising trends before they cross reportable thresholds
Pattern escalation
Flag rising trends before they cross reportable thresholds
Historical context
Compare current risk posture against prior periods instantly
Historical context
Compare current risk posture against prior periods instantly
Historical context
Compare current risk posture against prior periods instantly





Report Generation
Report Generation
Report Generation
Seamless vigilance review and regulatory reporting workflows
Detect potential reportability earlier and generate regulatory reports faster, with full traceability from intake to submission.
Reportability signals
Flag complaints that may require regulatory review
Reportability signals
Flag complaints that may require regulatory review
Reportability signals
Flag complaints that may require regulatory review
Linked investigation context
Connect complaints to CAPAs, servicing, and related records
Linked investigation context
Connect complaints to CAPAs, servicing, and related records
Linked investigation context
Connect complaints to CAPAs, servicing, and related records
Regulatory report generation
Generate MDR and vigilance reports in required formats
Regulatory report generation
Generate MDR and vigilance reports in required formats
Regulatory report generation
Generate MDR and vigilance reports in required formats
Full audit history
Track who reviewed, updated, and submitted each record
Full audit history
Track who reviewed, updated, and submitted each record
Full audit history
Track who reviewed, updated, and submitted each record
No more siloed data
No more siloed data
No more siloed data
Management-ready insights, always current.
Generate leadership-ready analysis without rebuilding charts or summaries by hand.
Live analytics
Always-current metrics without manual data assembly
Live analytics
Always-current metrics without manual data assembly
Live analytics
Always-current metrics without manual data assembly
Configurable dashboard
Customize key insights and trends
Configurable dashboard
Customize key insights and trends
Configurable dashboard
Customize key insights and trends
Audit-ready outputs
Reports backed by traceable source data
Audit-ready outputs
Reports backed by traceable source data
Audit-ready outputs
Reports backed by traceable source data
On-demand export
Download charts and tables for reviews or audits
On-demand export
Download charts and tables for reviews or audits
On-demand export
Download charts and tables for reviews or audits



Still Have Questions?
Still Have Questions?
Still Have Questions?
Still have questions? We’ve got you covered.
If it’s not covered here, reach out — or just try Hexa free and see for yourself.
Do we need to change how complaints are captured today?
No. Cytodyme builds on data that already exists and sits on top of your current systems. Complaints continue to be captured where they are today, while Cytodyme surfaces trends, classifications, and reporting views.
What systems can Cytodyme pull complaint data from?
Data can be pulled from sources your team already uses today, including systems like Salesforce and TrackWise, as well as other complaint and servicing tools.
Does Cytodyme replace our vigilance decision process?
Cytodyme is built to support post-market surveillance, complaint handling, and vigilance workflows in line with current regulatory requirements and guidance across regions.
This includes frameworks such as FDA 21 CFR Part 803 (Medical Device Reporting), 21 CFR Part 820, and EU MDR and IVDR vigilance expectations.
The platform prepares teams for regulatory decisions by surfacing relevant signals, structuring data, and generating draft reports, so reviews and sign-offs can happen faster and with greater confidence. Final determinations and submissions remain with your team.
Can we control which codes or dictionaries are used?
Yes. Code suggestions can be based on the classification systems your team already uses, such as IMDRF or MedDRA, and confidence thresholds can be configured so lower-confidence suggestions require manual review.
Can this help us meet tight global vigilance timelines?
By flagging potential reportable events earlier and organizing the required information in one place, Cytodyme helps teams act sooner on time-sensitive vigilance requirements, including global reporting obligations.
Do we need to change how complaints are captured today?
No. Cytodyme builds on data that already exists and sits on top of your current systems. Complaints continue to be captured where they are today, while Cytodyme surfaces trends, classifications, and reporting views.
What systems can Cytodyme pull complaint data from?
Data can be pulled from sources your team already uses today, including systems like Salesforce and TrackWise, as well as other complaint and servicing tools.
Does Cytodyme replace our vigilance decision process?
Cytodyme is built to support post-market surveillance, complaint handling, and vigilance workflows in line with current regulatory requirements and guidance across regions.
This includes frameworks such as FDA 21 CFR Part 803 (Medical Device Reporting), 21 CFR Part 820, and EU MDR and IVDR vigilance expectations.
The platform prepares teams for regulatory decisions by surfacing relevant signals, structuring data, and generating draft reports, so reviews and sign-offs can happen faster and with greater confidence. Final determinations and submissions remain with your team.
Can we control which codes or dictionaries are used?
Yes. Code suggestions can be based on the classification systems your team already uses, such as IMDRF or MedDRA, and confidence thresholds can be configured so lower-confidence suggestions require manual review.
Can this help us meet tight global vigilance timelines?
By flagging potential reportable events earlier and organizing the required information in one place, Cytodyme helps teams act sooner on time-sensitive vigilance requirements, including global reporting obligations.
Do we need to change how complaints are captured today?
No. Cytodyme builds on data that already exists and sits on top of your current systems. Complaints continue to be captured where they are today, while Cytodyme surfaces trends, classifications, and reporting views.
What systems can Cytodyme pull complaint data from?
Data can be pulled from sources your team already uses today, including systems like Salesforce and TrackWise, as well as other complaint and servicing tools.
Does Cytodyme replace our vigilance decision process?
Cytodyme is built to support post-market surveillance, complaint handling, and vigilance workflows in line with current regulatory requirements and guidance across regions.
This includes frameworks such as FDA 21 CFR Part 803 (Medical Device Reporting), 21 CFR Part 820, and EU MDR and IVDR vigilance expectations.
The platform prepares teams for regulatory decisions by surfacing relevant signals, structuring data, and generating draft reports, so reviews and sign-offs can happen faster and with greater confidence. Final determinations and submissions remain with your team.
Can we control which codes or dictionaries are used?
Yes. Code suggestions can be based on the classification systems your team already uses, such as IMDRF or MedDRA, and confidence thresholds can be configured so lower-confidence suggestions require manual review.
Can this help us meet tight global vigilance timelines?
By flagging potential reportable events earlier and organizing the required information in one place, Cytodyme helps teams act sooner on time-sensitive vigilance requirements, including global reporting obligations.