AI-Powered Productivity
Always-on
Post Market Visibility
One connected system to monitor real-world signals, review evidence, and generate the post-market reports regulatory and quality teams are responsible for
See Risk as it Develops.


Live analytics
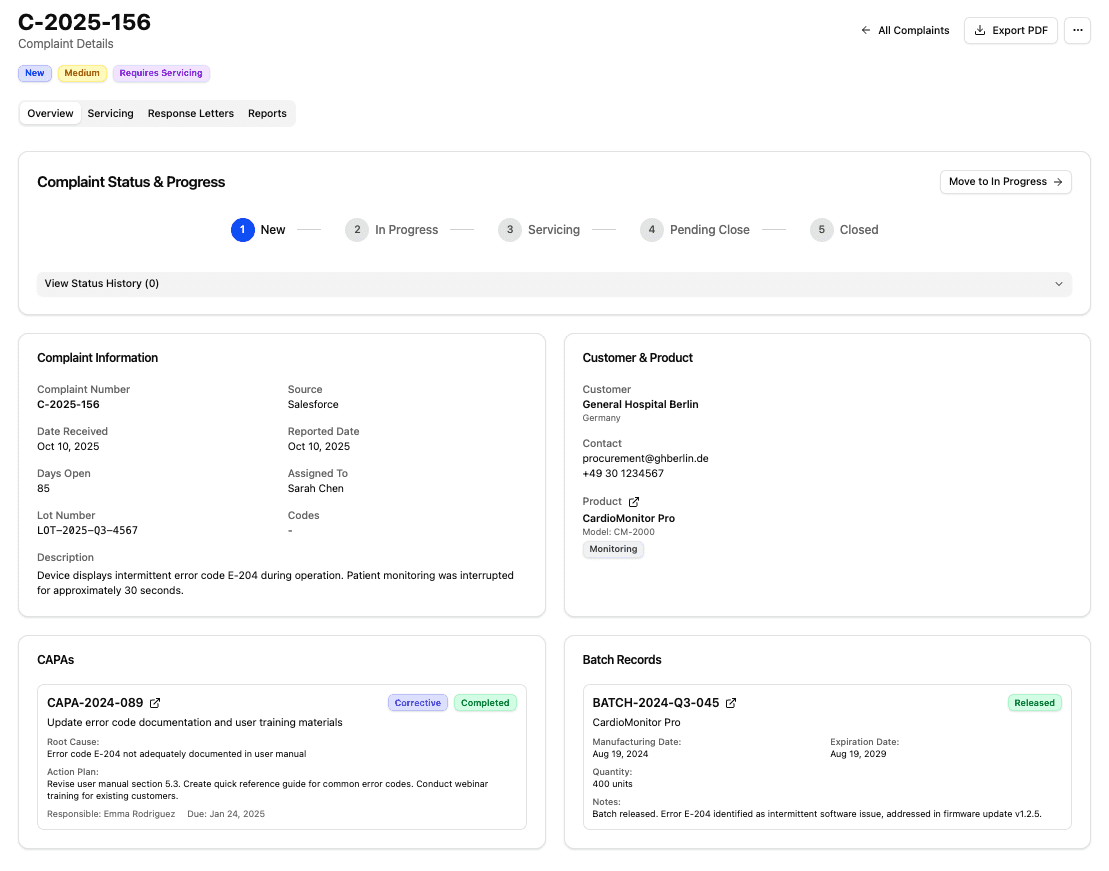
Turn complaint data into actionable safety signals.
Capture complaints from existing systems, assess risk and reportability, and manage investigations in one place as data accumulates over time.
Multi-source intake
Pull complaints from tools teams already use today
Multi-source intake
Pull complaints from tools teams already use today
Multi-source intake
Pull complaints from tools teams already use today
Vigilance workflows
Track status, investigations, and related records in one view
Vigilance workflows
Track status, investigations, and related records in one view
Vigilance workflows
Track status, investigations, and related records in one view
AI-assisted coding
Isolate trends by device, SKU, or configuration
AI-assisted coding
Isolate trends by device, SKU, or configuration
AI-assisted coding
Isolate trends by device, SKU, or configuration
Live risk signals
Severity and frequency update as new complaints arrive
Live risk signals
Severity and frequency update as new complaints arrive
Live risk signals
Severity and frequency update as new complaints arrive
Set it, and forget it literature surveillance
Set it, and forget it literature surveillance
Set it, and forget it literature surveillance
Monitor published evidence without manual screening.
Continuously monitor relevant literature, automatically screen results against defined criteria, and reuse evidence across post-market activities.
Unified search
Query PubMed, Google Scholar, and more at once
Unified search
Query PubMed, Google Scholar, and more at once
Unified search
Query PubMed, Google Scholar, and more at once
Protocol-based screening
Auto-screen abstracts using defined inclusion criteria
Protocol-based screening
Auto-screen abstracts using defined inclusion criteria
Protocol-based screening
Auto-screen abstracts using defined inclusion criteria
Audit-ready outputs
Reports backed by traceable source data
Audit-ready outputs
Reports backed by traceable source data
Audit-ready outputs
Reports backed by traceable source data
On-demand export
Download charts and tables for reviews or audits
On-demand export
Download charts and tables for reviews or audits
On-demand export
Download charts and tables for reviews or audits



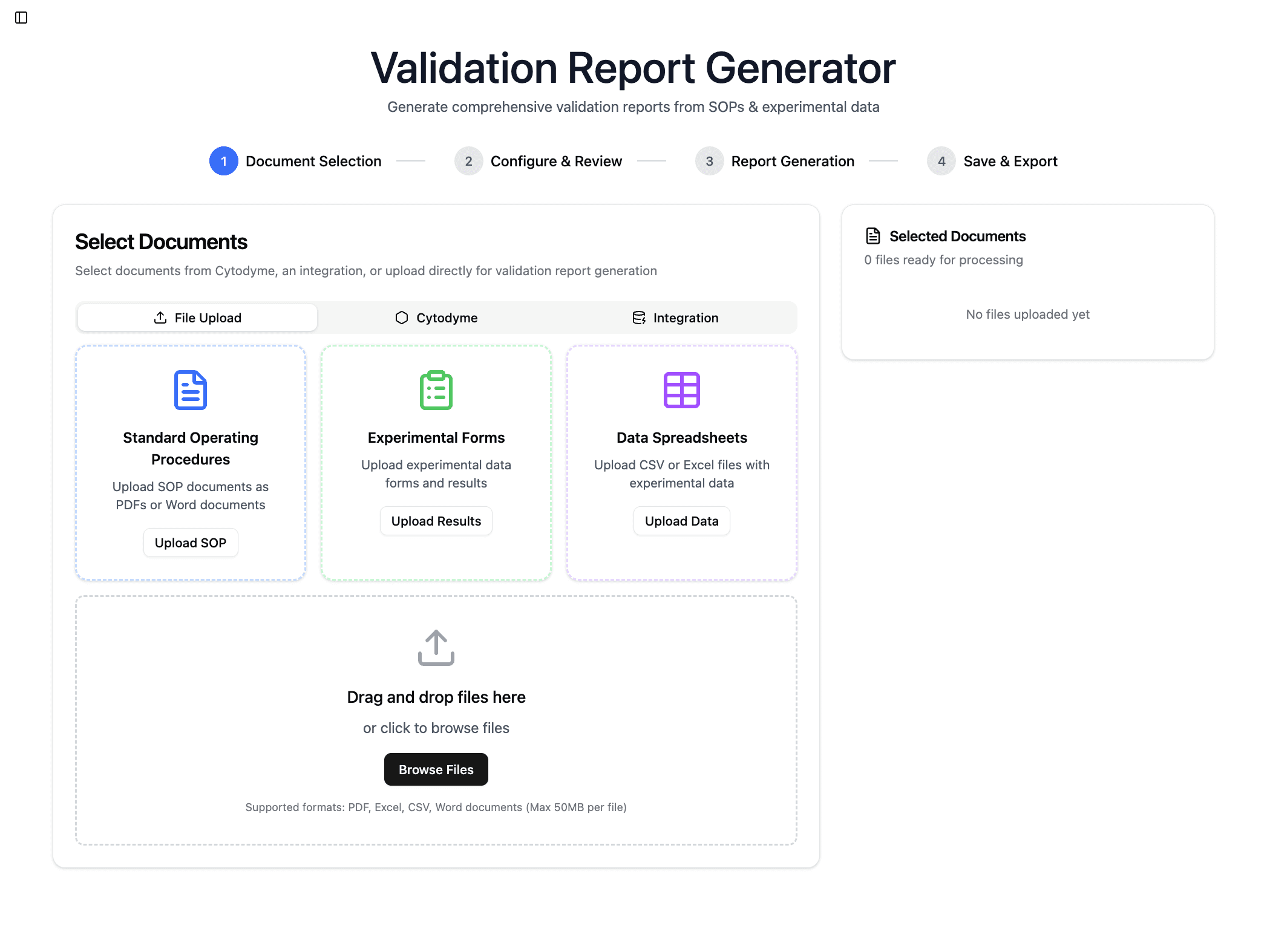


Pain-free report generation
Pain-free report generation
Pain-free report generation
Draft regulatory reports from traceable source data
Automatically generate structured drafts for required post-market reports using connected complaint and literature data, aligned to current standards and ready for team review and submission.
Automated PMS Reports
Generate core PMS reports like PSER, PMPF, and CER
Automated PMS Reports
Generate core PMS reports like PSER, PMPF, and CER
Automated PMS Reports
Generate core PMS reports like PSER, PMPF, and CER
Traceable inputs
Every section links back to its source data
Traceable inputs
Every section links back to its source data
Traceable inputs
Every section links back to its source data
Linked Changes
Changes in source data automatically surface affected report sections
Linked Changes
Changes in source data automatically surface affected report sections
Linked Changes
Changes in source data automatically surface affected report sections
Review before submission
Teams control final content and approvals
Review before submission
Teams control final content and approvals
Review before submission
Teams control final content and approvals